Garmin appears to have included ECG functionality within their recently released Venu 2 Plus smartwatch, albeit without acknowledging its existence or including the feature at launch. This is notable because there’s currently no Garmin wearable with ECG functionality on it, well, at least officially anyway. Still, Garmin’s shift towards medical devices and specifically ECG functionality wouldn’t necessarily be a surprise. Last spring, Garmin quietly began a clinical trial (2021) to test ECG-related features “derived from a Garmin wrist-worn, consumer device”.
The presence of an ECG diagnostics hardware diagnostics app on the Venu 2 Plus, combined with Garmin’s clinical trials testing, indicates the company is clearly working towards a goal of launching ECG functionality in their wearables. Of course, the timing of that is still unknown – as well as whether or not the Venu 2 Plus will *ever* officially gain ECG functionality.
When the company launched the Venu 2 Plus this past January, its core new features were a speaker/microphone, with calling-related functions. Omitted from that list, was any mention of ECG functionality – or even future ECG functionality. The fact that we’re seeing it now is largely due to a seemingly accidental act that left it in the diagnostic menus on units that were produced prior to February. Garmin has since removed that diagnostic menu in a firmware update, though it’s highly doubtful they’ve removed the actual ECG hardware.
At present Garmin doesn’t officially acknowledge its existence in the unit (more on that later in the post), but does acknowledge the clinical study. Of course, it kinda has to acknowledge that since it’s listed under their name on the US Government website. But again, we’ll circle back to that later in the post.
First Look at Garmin’s ECG:
The ability to see the ECG function is hardly a finished product. To be clear – everything you see in this post is the hardware test/diagnostics menu within the watch. This menu is normally reserved for Garmin technicians to troubleshoot various features. It includes diagnostic pages for sensors, accelerometers, NFC functionality, WiFi, Bluetooth, GPS, displays, etc… Generally speaking, if you’re using that menu – something has gone wrong and you’re trying to determine whether the hardware is the cause. It’s available on every Garmin watch and has been for probably a decade now.
However, what’s not been there is a new ECG diagnostic page. That was accidentally discovered by a user on the Garmin Forums back in early February. However, their experience there was short-lived, without any further documentation or photos. When they went back to access that feature later in the day, it was no longer there. That’s because, by that point, the watch has updated its firmware to the most recent version, which meant the option went away (it appears firmware version 8.05 in late January removed it – prior to the Garmin Forum post).
You see, Garmin, like most companies, manufactures hardware well before products start shipping to you. Apple, GoPro, and countless others do the same. It allows them to stockpile hardware while they finalize the software. This is often 2-4 months ahead of release. When these units are made, they need some sort of software on them so that when you unbox them for the first time, they’ll pair up to your phone and get updated to the current firmware. Ironically, I just posted about this concept a few days ago.
Given my existing Venu 2 Plus had long since updated to the most recent production firmware and removed the feature, I did what any normal person would do – I ordered another new Venu 2 Plus off of Amazon, and it arrived the next day. I then opened it up and without pairing to anything, went into the diagnostics menu. It showed the factory-made firmware version of 6.05 – so plenty old to still contain the feature. And sure enough, just two taps through the menu pages later was the ECG diagnostic menu:

Now to reiterate again, this isn’t meant to be a pretty display of your ECG. It’s a hardware diagnostic test screen, so it’s displaying the core data that Garmin needs to troubleshoot/test the ECG hardware itself. Undoubtedly, the normal end-user feature would be prettier and have pretty red ECG lines, warnings, usage instructions, and more. Why red lines? Because obviously, it has to be red. Any other color means it’s clearly fake.
The way the ECG app works is that you place your opposite thumb and index finger on the bezel, and within about 1-2 seconds it’ll start reading your ECG – taking a few more seconds to stabilize. The moment you remove your fingers from the bezel, it stops. This is essentially the same as how other companies have implemented it. With some companies (like Apple), you touch the digital crown instead, but the concept is the same. By using your opposite wrist/hand, you complete the required electrical circuit.

Playing around with hand position appears to relatively easily impact accuracy, as does playing around with one finger versus two fingers on the bezel. Certainly, this diagnostics software is likely 5-7 months old, and was never intended to do anything more than show raw sensor data. Just like all the other service menu pages do, for things like accelerometer, heart rate, Bluetooth, WiFi, GPS, and more.
Nonetheless, it’s clearly producing a valid-looking ECG. And given that Garmin completed a 568-person clinical trial last October with at least some Garmin-made ECG hardware, it’s likely by this point Garmin knows’ what they’re doing here. Here’s a look at how that ECG wave pattern looks compared to an Apple Watch Series 7, worn back to back (one after another):
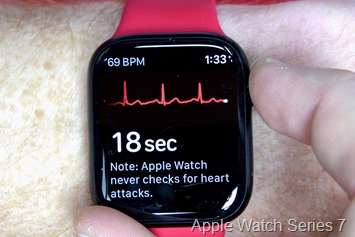
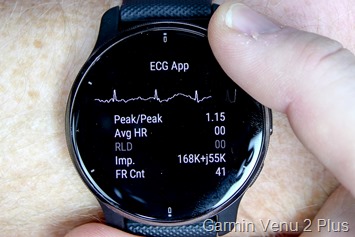
Now normally, most watches that feature ECG functionality also allow you to record the ECG trace and then export the results to send to a doctor. They also typically monitor/check for certain conditions, like atrial fibrillation, which is an irregular pattern. They NEVER check for whether a heart attack is occurring.
However, given this is in the diagnostics menu and not the normal feature, we don’t get that here. Undoubtedly, that’d be in any normal ECG feature down the road (because it’d be pointless without it), so we’ll have to see on that – if and when Garmin makes a feature out of this. And in fact, in Garmin’s clinical trials they even outline what features they’re trying to complete. So, let’s dig into that.
The Clinical Trials Study:
Last Spring Garmin recruited for a US clinical trial. As by law, any clinical trial in the US that’s done on people must be registered and listed publicly on the ClinicalTrials.gov database/website. That government organization isn’t quite the FDA, but has loose ties to it. It’s those clinical trials that are then used as the basis for submission to regulatory agencies like the FDA (in the US), or other medical regulatory bodies around the word. Garmin’s submission is here.
In Garmin’s case, they began the study last March 31st, 2021, and then concluded the response period on October 4th, 2021. That means that during that timeframe they were actively gathering data from real-world humans. Then last month (February 2022), they filed an update to the study indicating they did indeed conclude data collection last fall, as well as the final number of participants.
In Garmin’s case, they had initially filed the trials to include 460 participants, however, by the end, they had included 568 participants. In talking to various industry folks, that’s pretty normal, as participants that were initially included are occasionally removed from the study later on. That can be because the individual might not have met the criteria (more on that in a second) upon further inspection, or perhaps there were protocol errors in their sample. You’d expect to see some increase between the projected number and the final number.
The study’s purpose was, in their own words:
“to confirm the Garmin ECG (electrocardiogram) software algorithm can detect and classify atrial fibrillation and normal sinus rhythm on single lead ECG data derived from a Garmin wrist-worn, consumer device. The study will also confirm the software’s ability to create a Lead I ECG that is clinically equivalent to a reference device. The Garmin ECG software is not a diagnostic system and is intended for informational purposes only.”
In other words, they wanted to:
A) Validate the accuracy of the ECG data itself
B) Validate the accuracy of their software identifying atrial fibrillation and a normal sinus rhythm
Basically, they were aiming to have the same baseline validation and features that Apple, Samsung, and others do.
In order to do this, as the study outlines, they recruited through their partner research/medical organizations two different groups of people. Those with known Afib (atrial fibrillation), and those with a normal sinus rhythm. And they did this across 6 different sites in the US. It’s this two-group recruitment that can lead to differences in the final projected number, when for example someone says they have Afib, but upon doing a reference ECG with a doctor during the trial, they actually don’t. Thus, additional participants have to be identified.
The study’s execution was done by six different sites, though some of them are part of the same organization.
A) Hope Research Institute (Phoenix, AZ)
B) MedStar Washington Hospital Center (Washington DC)
C) HealthEast (St. Paul, MN)
D) Northwell Health North Shore University Hospital (Manhasset, NY)
E) Northwell Health Lenox Hill Hospital (New York, NY)
F) MedStar Health Cardiac Electrophysiology at Fairfax (Fairfax, VA)
Garmin themselves of course commissioned the study, and in particular, their health division. I thought it was notable that the application actually listed the names and titles of the Garmin people in charge of it. That itself wasn’t notable, but some rather the fact that Garmin now actually has someone with a job title of “Clinical Research Manager” was interesting (and her background at other organizations over the past decade is super interesting in microbiology R&D as well as managing other clinical trials and research). This makes sense, given that Garmin needs to have people with experience in the fields. Other names listed on the study also have experience in these areas.
The initial study documents do outline their exact protocol for each test participant:
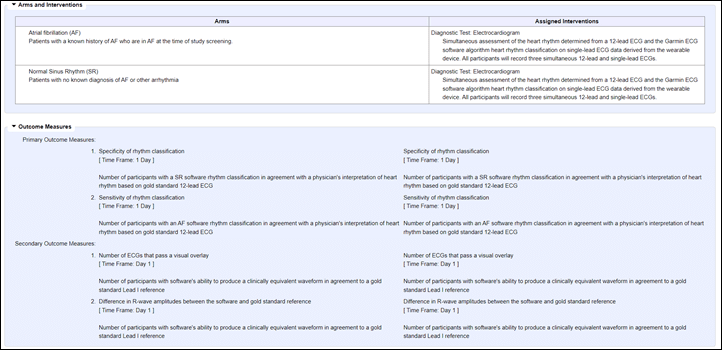
In a nutshell, the study collects data from a reference device, then collects data from a Garmin watch. Then they’re looking to understand whether or not the software correctly predicts the rhythm classification (Afib or normal). They’re also looking at secondary outcomes that validate that the pretty graph that you see is actually medical-worthy and that a doctor looking at it can correctly identify the rhythm classification. Finally, they’re looking to ensure that the R-Wave (pretty graph) matches that of their reference device.
At this point, Garmin has until later in the year to submit the final results of that study to the government. At present they haven’t uploaded that, which is normal. Most tech companies in this ECG space don’t typically upload those final results until they gain FDA approval.
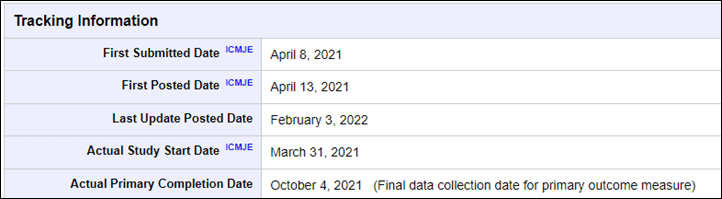
Still, the act that they updated the official file though on February 3rd, 2022, clearly indicates things aren’t dead here. Looking at the timeframe for the study, it was a bit longer than Apple’s was for their study (keep in mind Apple did this in 2018, pre-covid, which undoubtedly would have slowed down many aspects of this). Both companies had about the same number of people in the study, and the same number of sites (in fact, even sharing some sites/organizations).
Keep in mind though, the conclusion of the data-gathering phase back in October (for Garmin) is merely one step in a long road to FDA certification. In Apple’s case, their FDA certification took about 5-6 months after their study “primary completion date”. And again, it’s hard to reiterate the impact of COVID on delays to all processes here in 2022 versus 2018.
Going Forward:

That ultimately gets to the big question: Is an ECG feature coming to Garmin watches, or the Venu 2 Plus specifically? Well, it’d certainly seem that Garmin has been lining up their ducks for that to occur. Between the medical study last summer, the recent study filing document updates, and now the hardware clearly being inside the Venu 2 Plus – these are strong indicators.
But there are also reasons why it may never appear the Venu 2 Plus. First, it’s entirely plausible that the ECG components, or design of such, that Garmin put in the Venu 2 Plus didn’t meet their quality or accuracy levels for ECG. While that seems unlikely at that point, it’s certainly a scenario.
The ECG function here is classified as a medical device (under the FDA’s Software as a Medical Device program), it required approval by the FDA and similar organizations. Additionally, it typically requires that on a per-country basis. Some countries have their own bodies (e.g. the US), some have a central certifying body shared between countries (like in the EU), and some countries will simply follow certification by the US or EU. As we saw with Apple, Samsung, and others – those were a staged rollout, country by country. And some countries never received it, because the burden of getting medical device approval in a given country may not have been worth the effort for the number of devices in that country.
So, armed with all this curiosity, I simply asked Garmin. Here’s what Mary Woodbury, Garmin’s PR lead for their Wellness products/division (which the Venu series falls under) had to say:
“Garmin has conducted a clinical trial to assess the capability of our smartwatches to accurately detect the presence of AFib. The details of the study can be found on clinicaltrials.gov”
Which..is all they would say. They would not provide any comment regarding the existence of ECG features in the device itself – only confirming the clinical trial that existed. Their lack of comment would be in line with US FDA regulations that prohibit companies from discussing medical devices until FDA approval has been granted. In the case of Apple for example – they waited until they had actual approval from the FDA before announcing that ECG functionality would be coming to watches (even though there was another gap between the announcement of existence and implementation on consumers’ wrists). Whereas Withings, a French company, did not initially seek US FDA approval and thus pre-announced a year prior to the actual availability of the feature.
Remember, as advanced as Garmin is within the sports tech realm, they are a tremendously fiscal and legally conservative company. They virtually never pre-announce features. And in the last few years, they’ve shifted to not announcing products until they can ship immediately (versus announcing something coming in a few months). Them even acknowledging the existence of the ECG feature of this would land them in hot water with the FDA. Undoubtedly, a lot of four-letter words occurred in Kansas back in January when they realized this diagnostics menu was left in there.
However, in this case, their acknowledgment isn’t really necessary. They’ve acknowledged the clinical trial to detect AFib with an ECG in a Garmin wearable, and they’ve accidentally demonstrated that the Venu 2 Plus has capable hardware. The only things left to know are when and where. When will Garmin announce it, and to which country will it be available? Of course, there’s still the “if” factor. It’s entirely plausible that Garmin doesn’t request/receive FDA approval for this iteration. Thus, we may never see ECG on the Venu 2 Plus and certainly, I wouldn’t buy the Venu 2 Plus with that as a reason, since no promise (or even hint) has ever been made in that realm.
With that, thanks for reading!

